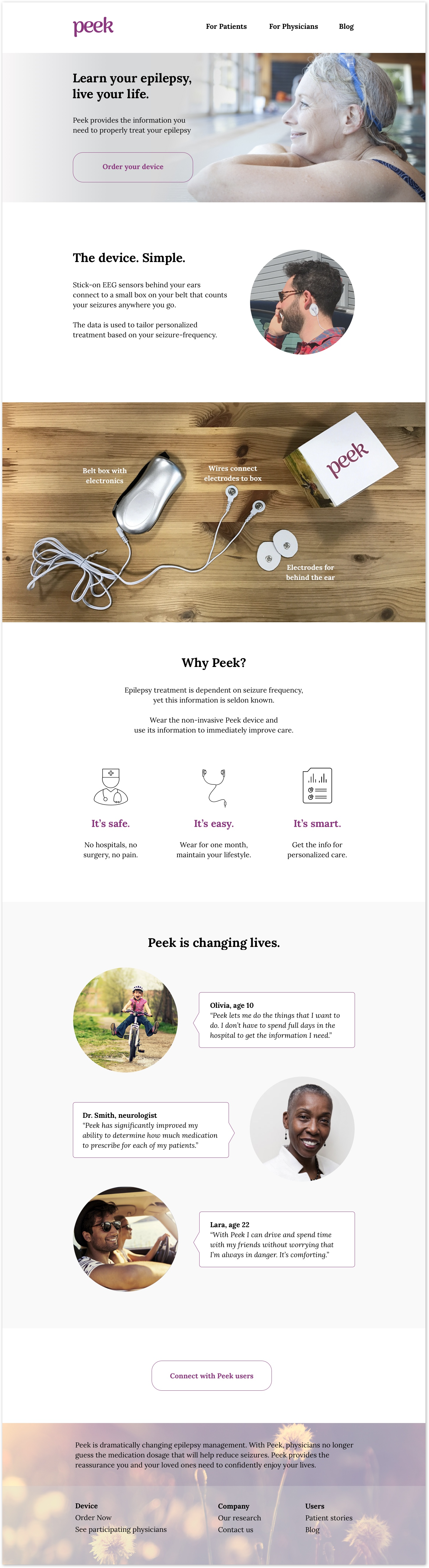
Designing a medical device for epilepsy treatment
The device has stick-on EEG sensors behind your ears that record and count your seizures anywhere you go. Results are delivered via mobile app.
Current hospital tracking method
The Challenge
How might we count how frequently someone has seizures without being in a hospital?
Epilepsy treatment is dependent on the frequency patients have seizures, yet getting this data requires brain surgery or spending days in a hospital hooked up to hundreds of electrodes. Our mission was to design a device to accurately count seizures during daily life, outside of a hospital.
Project Overview
Through Yale’s Center for Engineering, Innovation, and Design, I partnered with a team of engineers and two doctors at Yale Medical School on this hardware and software project. Leveraging new research from the medical school, we built a working prototype—a wearable device to track epileptic seizures. The device is not yet in market, though research continues at Yale. Following the product build, I designed the visual identity, website, mobile app, and packaging on my own.
My role: UX Designer & Product Manager
Deliverables: User research, concept development, market analysis, stakeholder mgmt, UX design, visual design and branding, mobile app design
Users: Epilepsy patients and their physicians
Team: Engineers – Levi DeLuke, Lauren Gardanier, Tammer Abiyu, Jason Allmaras and Physicians – Dr. Robert Duckrow, Dr. Jason Gerrard
Time period: 6 months, 2014
The Solution
My team and I built a physical prototype of the wearable device that was able to accurately detect changes in brainwaves using a device that did not require hospital equipment. It has three parts:
Sensors that stick on behind the user’s ears
Electronics box worn on the user’s belt that records brain activity
Wires that connect the sensors to the electronics box
I then designed the visual identity, landing page, and mobile app.
Landing page
Mobile app
After a month of wearing the device, patients can view their results through the Peek mobile app. They will see an overview and count of their seizures, and receive a personalized prescription.
Product Development Process
Our analysis of the existing epilepsy treatment ecosystem showed a huge gap in options—they were either accurate or easy to use. Our device needed to be both. We conducted many interviews to better understand both physicians' and patients' needs. Physicians needed accurate information so they could prescribe medication. Patients needed to reduce the frequency and severity of their seizures.
Research findings
Through our user research, we established the following priorities for our design.
Concept development
To identify the right form factor, we looked for inspiration in many wearable devices, and considered how we could get an electrode physically close to someone's brain—even by putting electrodes in a mouth retainer or a facial piercing. Given the trade-offs among the options—we quantitatively ranked each option against our functional requirements to find a balance between complexity, comfort, and ease. The area behind the ears came out as the winner through our quantitative evaluation.
Form factor analytical evaluation to determine where to put the electrodes
Usability testing
Finding optimal electrodes led us to experiment with many types—each requiring different application procedures from glue to adhesive. Immersing ourselves in wearing electrodes to the gym, in the shower, and to sleep taught us that there was a lot of variability in how the different types performed during normal activities. We learned that glue was too messy, and that some of the adhesives weren't strong enough. The best electrode had an easy stick-on application that could last several days and was flexible enough to fit comfortably on a moving body.
Engineering build
I project managed the engineering work—balancing the competing priorities of achieving the smallest circuit board design possible while keeping us on schedule. The boards needed to be able to record and count seizures, housed in a small box worn on the patient's belt. We started with an OpenEEG schematic for digital and analog boards. They had to shrink in size, and we iterated on the design to eliminate unnecessary parts of the electronics.
OpenEEG schematic for circuit board - lives inside belt box
Functional testing
To test, we soldered the components to circuit boards and recorded conductance and sensitivity.
With a working prototype, we visited the hospital to test its performance against clinical equipment. The same patient was hooked up simultaneously to our device and the clinical EEG machine to detect neural activity.
Our device worked! We proved the concept of a portable, non-invasive device to track seizures outside a hospital.
Neural activity output of our device compared to the hospital equipment
Visual Design and Brand Identity
For the device's visual design, I wanted to use soothing visuals to address the psychological distress associated with epilepsy. Tackling epilepsy challenges is hard work, and people need support and encouragement to feel empowered.
User research
I conducted a second round of user research, and these two women helped me identify the vibe I wanted the branding to achieve. Their openness and ability to find beauty and peace amidst a challenging situation was the inspiration for my visual design.
Moodboard
The board conveys an active, confident, and peaceful life. Overcoming challenges can lead to a greater appreciation for life–which is a hidden beauty, like this peacock feather, the flowers on a cactus, or the colorful inside of a geode. I wanted to bring this idea to the device's DNA.
Type and color studies
Purple is the color of the Epilepsy Foundation and thus helps to reinforce the connection to the medical community. I chose Arima Madurai as the logo font-face because of its friendly and fluid appearance, and Lora as the body font-face for its more traditional serif style for medical gravitas.
Style guide
The style guide portrays a simple, active and calm lifestyle.
Packaging
Solution and Customer Journey
The Peek device tracks seizures outside of a hospital—using only two electrodes connected to a belt box that records neural activity. We built a working prototype of the device, and I designed the user experience of the device, mobile app, and website, as well as the branding.
Step 1
An epilepsy patient orders the Peek device online through a desktop or mobile website.
step 2
The device arrives in a packaged cube. The patient applies the electrodes and snaps the wires to connect the electrodes to the recording box, which clips onto the patient's belt.
step 3
The patient wears the device for one month, maintaining their current lifestyle outside of the hospital.
step 4
After one month, the patient reviews their results through a mobile app and receives an updated personalized prescription.
Identifying business partners
Getting buy-in from doctors will be essential to the product's success. I used ArcGIS to find early partners to launch the MVP and collect feedback. Because epilepsy is not concentrated in a certain geographic location, I identified areas that were likely to have 1) more people, thus more people with epilepsy, and 2) overall higher spend on healthcare. I made the assumption that clinics in areas with these two characteristics might be more interested in working with us.
Next steps
The Peek device is not yet on the market. Medical researchers at Yale are working on solidifying the electronics and battery design before it's ready to be worn by a patient for a month.